那个问题让我想起,前几周有个比力瘦的学员说他之前的锻练让他吃高热量食物来增肌。
给学员摆设高热量食物为主,让我觉得那个锻练十分的业余,专业常识贫瘠。很显然那个锻练跟本问题下大大都人的观点一样,认为高热量饮食/瘦削不影响增肌,或者对增肌有利。
但是,支流学术界倾向于认为认为高体脂/瘦削对增肌的负面影响更大。
一、高体脂/瘦削可招致『合成代谢抵御』『合成代谢』是个健身术语,指人体操纵食物的营养物量合成本身组织的过程。
有良多因素能够招致人体合成代谢效能(能够粗略理解为增肌效能)下降,好比人变老肌肉的胰岛敏感性降低会越来越低[1][2],肌肉摄取和操纵的葡萄糖会越来越少,肌肉合成代谢率越来越低[3][4],合成代谢率越来越高。
科学上把那一类降低合成代谢效能的现象称为『合成代谢抵御』[5][6][7](anabolic resistance)。
合成代谢抵御被认为是人类衰老过程中肌肉量逐步削减的重要原因[8][9][10][11];许多研究发现中老年人的根底卵白量合成率较低[12][13][14][15][16],好比Benjamin等人对差别年龄人群的察看发现,年轻受试者餐后的肌卵白合成反响高于老年受试者[17]:
图1然而,本文讨论的不是衰老招致的合成代谢抵御,而是体脂高能否影响增肌。
现有的证据表白,体脂较高或者瘦削,跟衰老一样,会招致差别水平的合成代谢抵御,而且医学上不断认为衰老的肌肉和脂肪堆积的肌肉(脂肪堆积在肌纤维内、肌纤维间)是类似的。
Guillet等人的综述表白[18],瘦削者肌肉内的脂肪积累,招致骨骼肌的胰岛素抵御,引起骨骼肌吸收和处置葡萄糖的才能下降,以致骨骼肌的力量和量量受损;
并且那种情况不单单发身在人类身上,似乎高档哺乳动物也是如斯:Jason等人对动物离体肌肉研究发现[19],瘦削动物(通过高脂肪饮食喂食)在16周运动后的下肢肌肉(比目鱼肌、趾长伸肌)内,肌卵白的表达显著降低:
图2(部门需要复杂解释的数据没放上来)高热量饮食确实对增肌有帮忙,但是同时也有坏处,那两件事能够同时发作,其实不矛盾;那就像借高利贷做生意能够敏捷获得一笔资金,害处则是将来要还的太多,益处和害处是能够同时存在的,以至世界良多工作也都是如斯。
二、高体脂/瘦削与合成代谢抵御之间的重要桥梁:胰岛素抵御谈到合成代谢,就不能不提到胰岛素,它是人体最重要的合成代谢激素之一。胰岛素发现于1921年[20][21],次要感化是促进细胞摄取营养物量。
胰岛素从血液运输并抵达肌细胞外表后[22],与受体连系[23][24][25][26],引发一系列细胞内的生物化学信号[27][28],促使葡萄糖转运卵白往细胞外表挪动,细胞摄取葡萄糖,并将其合成为糖原。
图3:胰岛素感化机造关于增肌者来说,胰岛素十分重要:
胰岛素是激素,激素与营养、训练一样,都有 *** DNA表达的感化;抗阻训练后的肌肉增长具有胰岛素依赖性,胰岛素程度高时,训练卵白合成速度和DNA翻译效率显著增加[29];胰岛素还能与氨基酸协同感化,产生1+1>2的效果,放大卵白量合成的效率[30][31];良多研究发现,打针亮氨酸+胰岛素,比零丁打针此中一种,对血液中的卵白合成信号加强更多;胰岛素能扩张毛细血管,那是通过 *** 一氧化氮合成来实现的[32];毛细血管扩张的成果是肌细胞得到更多的血流量[33][34][35]和营养物量,那关于增肌和肌肉的安康来说当然很重要;在某些情况下,胰岛素的血管扩张效果会很强,例若有研究发现进食 *** 的胰岛素排泄可使安康人的骨骼肌微血管血流量增加50-80%[36]——并且我们应当意识到,那种效果是相对耐久的,往往会持续多个小时,那比起训练形成的短期效果来说,现实意义更大,因为有许多关于激素的研究都发现训练中短暂升高的各类合成代谢激素与增肌之间的关系十分小,几乎能够忽略不计。胰岛素对增肌来说是如斯的重要,以致于,若是利用抗胰岛素抗体,消弭胰岛素的感化,会大幅削弱以至完全消弭肌肉增长的 *** 效果[37][38][39][40][41];与胰岛素的效果相对应,各人应该都晓得胰岛素抵御。胰岛素抵御指胰岛素促进细胞摄取营养素的才能下降[42][43][44][45],就像原来1个单元胰岛素就能驱动1个单元葡萄糖被细胞摄取,如今要1.5以至2个单元胰岛素才行了。
在胰岛素抵御的情形下,就算吃了足够的碳水,肌细胞也无法摄取足够的葡萄糖。有数据表白,胰岛素抵御的小鼠肌细胞的Glut-4比对照组削减50%摆布[46]。
胰岛素发作抵御,胰腺只能被迫排泄更多的胰岛素,就会构成高胰岛素血症,那是糖尿病发作的前兆;若是继续恶化,胰岛素的感化会越来瓯越弱,人的骨骼肌吸收营养物量的才能会越来越低,肌肉体积就会逐步缩小。
瘦削和过量脂肪的摄入,都可形成差别水平的胰岛抵御。
在任何一般医学/营养学教材上,2型糖尿病的饮食因素都有高热量、高脂肪几个字;持久高热量、高脂肪饮食,甚至带来的高体脂、瘦削,会促进胰岛抵御和糖尿病,那是医学共识,也是常识。
并且现有证据表白,哪怕只是短期高热量/高脂肪饮食也可加重胰岛抵御。
2009年,Charlotte等人研究了短期过量脂肪摄入对男性胰岛敏感性降低的影响[47],26名年轻安康的男性停止持续5天高热量饮食(总热量是日常平凡的150%,且有60%总热量来自脂肪);5天后,受试者空腹肝脏葡萄糖产量增加26%,而空腹血糖升高是胰岛抵御的标记[48],
图42020年的一项研究[49]察看了一般人持续7天摄入超出总热量47%的高热量高脂肪饮食,此中脂肪占总能量64%,成果是人类胰岛素程度升高19%,胰岛敏感性降低-26%,表白发作了胰岛抵御。
所以,文章开头那种教学员用高热量饮食来脏增肌的思绪,本色上就是把短期效果超出于学员持久安康的做法,既缺乏专业常识和医学常识,也缺乏职业道德。
至于为什么瘦削和高体脂会促进胰岛抵御以至可能开展为糖尿病,我们也做一些简单的解释:过量热量摄入/过高的体脂/瘦削通过慢性炎症来损害胰岛素信号,促进胰岛抵御。
我们之前的文章说过,脂肪组织不只是脂肪滴的容器,也是调理内排泄的器官,好比内脏脂肪排泄各类促炎物量,如白介素IL-6、C-反响卵白CRP[50]等。
瘦削、摄入的总热量过多,都能上调炎症,形成胰岛抵御,那早就被频频证明过[51][52][53][54][55][56];动物[57]和人类[58][59][60][61][62][63][64]过量进食都形成空腹胰岛素程度升高和胰岛抵御。
除了过量饮食招致炎症,肠道菌群的相关研究也提醒了一些眉目。
研究摄入较多饱和脂肪会改动肠道菌群[65],增加吸收处置脂肪酸的菌群数量[66][67],增加了肠道对糖多脂(lipopolysaccharide )的通透性[68][69],在瘦削和代谢综合征人群中已经察看到了血液糖多脂程度增加[70]。
糖多脂从肠道大量进入血液,在血液中程度升高,然后激活TLR-4受体[71],一种由巨噬细胞排泄的炎症信号因子,从而激活慢性炎症信号[72][73][74][75][76];慢性炎症通过一系列复杂的生化信号,引起胰岛素信号受损,招致胰岛抵御发作。
胰岛素抵御的成果是多方面的,除了我们上面说的各类益处享受不到,好比促进肌肉血流量增加/血管扩张、促进卵白量合成等,还有个最关键的:招致肌肉的燃料改动[77][78],即从『烧葡萄糖』转为『烧卵白量和脂肪』[79][80],于是肌肉体积和力量发作丧失,也就成了合成代谢抵御。
有些同窗可能会有疑问,既然体脂高的人有足够的脂肪供能,为什么还要燃烧卵白量呢?
那个问题我们之前的文章已经解释过:脂肪/酮体[81][82]都不克不及零丁供能[83][84][85],它必需要碳水/葡萄糖、或者是唐代谢中间产品共同[86][87][88][89],才气氧化供能。
传送门在此:
脂肪燃烧需要碳水吗?915 附和 · 65 评论答复回到本文,若是胰岛敏感性降低,肌肉获得的碳水/葡萄糖不敷,就只能把肌卵白转化为碳水/糖类衍生物[90][91],来共同脂肪燃烧——那就是为什么糖尿病人城市大量丧失肌肉,变得瘦削。
三、高体脂/瘦削按捺肌卫星细胞激活我之前的文章已经说过,增肌的核心不是『损伤/扯破』,而是DNA对外界的机械 *** 做出的反响。训练/饮食/激素都以细胞信号的体例传递到DNA上,DNA转录为RNA,翻译为卵白量。
图5对此有疑问的同窗能够归去补课。
健身增肌的原理是什么?1517 附和 · 112 评论答复没有疑问的同窗继续听。
DNA转录和翻译为卵白量,是有空间范畴限造的;因为人体的肌细胞是个长长的管子,是个多核细胞,每个细胞只能在它四周产生新的卵白量[92][93],那个范畴叫做构造域[94][95]。
图6:构造域因而,细胞核的数量越多,增肌效果就越好。
Adam等人1996年颁发了一篇名为《骨骼肌肥大过程中DNA含量与卵白量积累的关系》的论文指出:DNA的总重量与肌肉的量之间,存在很强的正相关性。
图7:肌肉量与DNA总量之间的正相关性那些履历了训练后增肌效果极佳的人,他们的细胞核比效果一般/效果差的人多好几倍。
图8:增肌幅度与细胞核(DNA)数量之间的关系那些我们以前也讲过了:
肉崽:训练潜力与遗传(三):肌肉程度与细胞核102 附和 · 23 评论文章细胞核的数量是增肌效果的核心;而细胞核的数量是能够增减变革的,此中一个重要的来源就是肌卫星细胞,它向肌纤维捐赠新的细胞核[96][97][98][99][100]。
所以在增肌过程中,肌肉体积增大,都陪伴肌细胞核增加[101][102][103][104];而且,肌纤维大小和肌核数量之间的比值,几乎老是连结恒定[105][106]。
那与本文的关系是:瘦削和高脂肪饮食按捺卫星细胞的激活。
一般情况下,卫星细胞的激活需要HGF(肝生长因子),HGF与脊髓上的连系后,会激活一种酶(SK1),那种酶负责启动并将卫星细胞活化[107]。
Donna等人察看发现[108],高脂肪饮食喂食的瘦削小鼠体内HGF信号改动,下调了卫星细胞的激活,并进一步招致肌肉的再生和修复削减。
原文中,CON是一般小鼠,DIO是饮食诱导的瘦削小鼠(Diet-Induced Obesity ),图中DIO小鼠的HGF显著削减:
图9当然,瘦削形成骨骼肌合成代谢抵御、修复延迟的证据和原理还有良多,本文因为篇幅关系不克不及继续说了,先就说到那里。下面我们进入下一个话题:瘦削形成骨骼肌力量、功率和收缩才能的下降。
四、高体脂/瘦削招致骨骼肌收缩才能下降在瘦削对肌肉的影响方面,先前的研究集中在肌肉的绝对力量或功率方面[109][110][111]。
那些研究已经表白,瘦削会增加体重,招致那些支持体重、维持身体姿势的肌肉增大,但是对那些不参与支持体重、维持身形的肌肉则没有影响[112]:如Rolland等人发现,瘦削老年女性膝伸肌的绝对力产生才能增加,但握力没有显著变革[113]。
后来的研究起头存眷单元体积的骨骼肌力量。
那些人类研究一致的表白,瘦削招致单元体积的肌肉收缩效能下降[114][115][116][117][118],出格是骨骼肌筋膜下、肌纤维之间、和肌纤维内的脂肪—即『肌肉间脂肪—IMAT』[119][120]的积累对肌肉功用有明显的损害。
Choi等人他们察看了13名一般体重白果和21名瘦削白果[121],阐发了每个受试者的至少80根肌纤维,发现瘦削组单个肌纤维的绝对力量、相对力量、收缩速度均低于一般体重组;那个研究还确认,在瘦削白果中,肌纤维内脂滴的大小与肌纤维的工做才能(收缩力量和速度)呈强烈的反比关系。图10Miyatake等人研究了357名20-79岁的瘦削受试者中,并与年龄和性别婚配的1683名非瘦削对照受试者停止比力,瘦削受试者的腿部力量与体重的比值明显低于对照组[122];Hulens等人研究了173名瘦削女性和年龄婚配的80名一般性,校正后,瘦削女性的所有力量丈量值至少比一般女性低6%(但躯干屈曲除外)[123];Paolillo等人研究了45名瘦削和非瘦削女性,测试了他们的更大肌肉力量(峰值扭矩),当数据按体重和瘦体重停止尺度化后,瘦削女性的值明显低于非瘦削组[124];不但人类,动物研究的结论也类似。
对动物离体肌肉研究发现[19],瘦削动物(通过高脂肪饮食喂食)在16周运动后,肌肉更大力量(强曲收缩扭矩,图4)和功率(图5)与精瘦的对照组比拟较低;
图11(部门需要复杂解释的数据没放上来)图12(部门需要复杂解释的数据没放上来)研究者揣测,体脂高和瘦削招致肌肉收缩效能降低的原因,可能是瘦削者激活的横桥数量较少、或者单个横桥产生立的力较低[125],或者瘦削者的肌原纤维数量较少[126];
还有些研究者思疑是肌细胞内角大的脂滴构成了电阻,障碍了动做电位的传导,从而降低了肌肉收缩速度[127][128]。
五、高体脂/瘦削降低肌肉的抗委靡才能说起抗委靡,可能良多在意肌肉和力量的人觉得那不是他们的首要目的;但若是我换个说法,说瘦削会削减训练容量,可能良多人就会睁大眼睛了。
Paolillo等人研究了45名瘦削和非瘦削女性,当数据按体重和瘦体重停止尺度化时,瘦削女性的抗委靡才能明显低于非瘦削女性[124];
Nicola等人[129]对20名瘦削(BMI41)精瘦(BMI23)的受试者停止了股四头肌更大伸膝力量的试验,50次伸膝训练后,两组的力量(扭矩)都显著下降,精瘦组下降50.6%,瘦削组下降了63.5%,表白瘦削者在的肌肉委靡抗性较差;
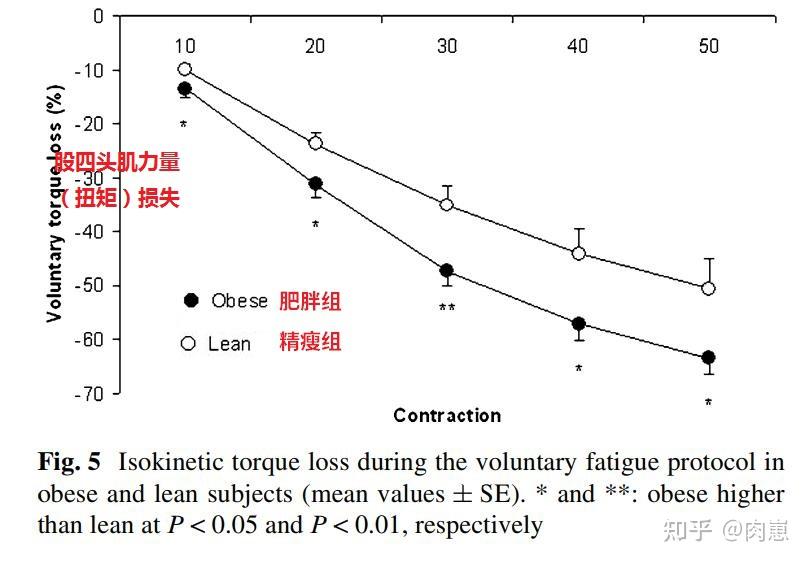 图13
图13动物研究的结论跟人类研究不异。Jason等人陈述[19],瘦削动物(通过高脂肪饮食喂食)在16周运动后,肌肉抗委靡才能(在高功率下维持运动的时间)显著低于精瘦的动物:
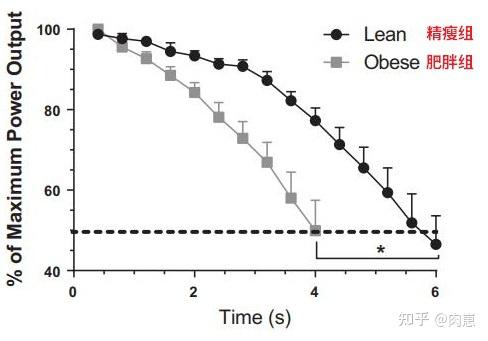 图13
图13瘦削招致骨骼肌抗委靡才能下降的原因,目前的研究已经总结出了多种机造。
一种是瘦削招致骨骼肌的能量供给受损,从而降低了骨骼肌的耐久性。
骨骼肌中的能量来自葡萄糖和脂肪酸合成,那通过脂联素(Adiponectin )来调理;脂联素激活另一种重要的酶—AMP激酶[130],介导葡萄糖/脂肪酸等能源物量的氧化和操纵。
而瘦削,出格是骨骼肌中的脂肪积累会降低脂联素程度,从而按捺细胞的能量获取[131],招致骨骼肌的收缩才能和接受训练量的才能下降。
还有一种是瘦削招致骨骼肌的收缩-舒张效率下降,浪费了能量,降低了接受训练量的才能。
各人都晓得,骨骼肌的收缩和舒张依靠肌丝滑行[132][133][134][135]。
(1)当我们主动用力的时候,神经系统向肌纤维释放生物电[136][137],那些电流沿着细胞膜传遍整个肌纤维[138][139][140],然后 *** 了肌浆网[141][142];
(2)肌浆网在生物电 *** 下释放钙离子,钙离子与粗/细肌丝上的肌钙卵白连系,招致其构象变革[143][144][145],于是粗细肌丝之间的横桥由锁定形态变成解除形态[146][147];
(3)横桥的锁定被解除后,横桥拖动粗细肌丝相对滑动、肌肉收缩[148][149],其能量来源于横桥上的ATP袋,ATP就在此中水解释放能量。
(4)我们不消力了,钙离子就会逐步回到到肌浆网[150][151][152][153],肌肉舒张;留意,那一过程是需要消耗能量的。
也就是说,生物电并非在间接 *** 肌肉收缩,而是 *** 肌浆网释放和收受接管钙离子;钙离子释放和收受接管是肌肉收缩的本色。
而瘦削被证明对钙离子的处置有不良影响:
Bruton等人发现,瘦削动物的肌纤维对钙离子处置存在异常,处置效率较低,从而招致那些动物的肌肉发作过快的委靡[154];Jolita等人对喂食高脂肪食物的小鼠察看发现,他们的肌肉败坏时间比对照组慢大约30%摆布[155];因为肌肉舒张要消耗ATP,更低的肌肉舒张效率可能招致更多的能量浪费,因而瘦削动物的抗委靡(抗容量)才能较低;体脂高和瘦削对增肌、力量、训练量的负面影响原因良多,本文因为篇幅有限,暂时只列出那么几个(若是写更多,良多读者底子看不完)。
启 示:1. 体脂高/瘦削会招致合成代谢抵御,我们应该先减脂,再增肌;
2. 增肌过程中不要纵容体脂飙升,更好不要脏增肌;
3. 逃求增肌的人应当按期减脂,而不是一条路走到黑,增到十分高的体脂才减;
4. 喜好逃求力量的训练者应当连结中等略低的体脂,因为体脂高/瘦削削减肌肉力量;其实职业举重和力量举,均匀体脂也是10%摆布(跟健美运发动上台类似),当然不包罗120+级别;
5. 若是希望提拔本身的训练量和抗委靡才能,恰当削减体脂是一个明智的做法;
6. 慢性炎症会增加睡眠时间(并非睡太多招致疾病,而是有疾病、有慢性炎症的人会睡得多),因而恰当削减体脂还能减轻体内的慢性炎症,减轻委靡和嗜睡感,节约时间;
7. 除了瘦削以外,贫乏活动和久坐也会促进合成代谢抵御,降低肌原纤维合成速度[156][157][158],例如安康男性停止14天腿部石膏固定,卵白量合成旅削减了均匀31%[159];那申明增加日常体力活动量,而不是久坐,关于肌肉生长和代谢、关于制止合成代谢抵御有必然帮忙;
扩展阅读肉崽:力训研究所课程介绍
肉崽:为什么碳水和糖才是长胖元凶,明明脂肪热量更高啊?
肉崽:健身最隐讳什么?
肉崽:有氧运动会掉肌肉吗?
肉崽:脂肪在运动过程中是怎么改变为能量的,人体消耗的脂肪是不是有位置优先级?
肉崽:高位下拉时若何制止前臂代偿发力?
肉崽:为什么共识认为精造碳水,如面食会招致瘦削?
肉崽:健身房开空调会影响减肥效果吗?
参考^Mirian Ayumi Kurauti 1, Gabriela Moreira Soares 2, Carine Marmentini 2, Gabriela Alves Bronczek 2, Renato Chaves Souto Branco 2, Antonio Carlos Boschero 2.Insulin and aging.Vitam Horm. 2021;115:185-219.^Jose A Morais 1, Kathryn Wright Jacob 2, Stéphanie Chevalier 3.Effects of aging and insulin resistant states on protein anabolic responses in older *** s.Exp Gerontol . 2018 Jul 15;108:262-268.^Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM & Rennie MJ (2005). Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 19, 422–424.^Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, Walker DK, Dhanani S, Volpi E & Ra *** ussen BB (2011). Aging impairs contraction‐induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet Muscle 1, 11. ^Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19(3):422–4. ^Katsanos C, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe R. Aging is associated with diminished accretion of muscle proteins after the ingestion of a *** all bolus of essential amino acids. Am J Clin Nutr. 2005;82(5):1065–73.^Burd N, Gorissen S, van Loon L. Anabolic resistance of muscle protein synthesis with aging. Exerc Sport Sci Rev. 2013;41(3):169–73. ^Evans W. What is sarcopenia? J Gerontol A Biol Sci Med Sci. 1995;50:5–8. ^WHO. [http://www.who.int/topics/ageing]. webcite. 2008.^Evans WJ. What is sarcopenia? J Gerontol A Biol Sci Med Sci. 1995;50:5–8.^Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol. 1995;50:11–16. ^Hasten D, Pak-Loduca J, Obert K, Yarasheski K. Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in 78–84 and 23–32 yr olds. Am J Physiol Endocrinol Metab. 2000;278(4):620–6. ^Balagopal P, Rooyackers O, Adey D, Ades P, Nair K. Effects of aging on in vivo synthesis of skeletal muscle myosin heavy-chain and sarcopla *** ic protein in humans. Am J Physiol. 1997;273:790–800. ^Welle S, Thornton C, Jozefowicz R, Statt M. Myofibrillar protein synthesis in young and old men. Am J Physiol. 1993;264:693–8. ^Welle S, Thornton C, Statt M. Myofibrillar protein synthesis in young and old human subjects after three months of resistance training. Am J Physiol. 1995;268:422–7.^Yarasheski K, Zachwieja J, Bier D. Acute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and women. Am J Physiol. 1993;265:210–4. ^Benjamin Toby Wall, Stefan H. Gorissen, Bart Pennings, René Koopman, Bart B. L. Groen, Lex B. Verdijk, and Luc J. C. van Loon.Aging Is Accompanied by a Blunted Muscle Protein Synthetic Response to Protein Ingestion.PLoS One. 2015; 10(11): e0140903.^C Guillet 1, A Masgrau, S Walrand, Y Boirie.Impaired protein metaboli *** : interlinks between obesity, insulin resistance and inflammation.Obes Rev. 2012 Dec;13 Suppl 2:51-7.^abc Jason Tallis,1 Cameron Hill,1 Rob S. James,1 Val M. Cox,1 and Frank Seebacher2.The effect of obesity on the contractile performance of isolated mouse soleus,EDL, and diaphragm muscles.J Appl Physiol 122: 170–181, 2017^Banting FG, Best CH, Collip JB, Campbell WR, Fletcher AA. Pancreatic extracts in the treatment of diabetes mellitus. Can Med Assoc J. 1922;12:141–146. ^Karamitsos D.T. 2011. The story of insulin discovery. Diabetes Res. Clin. Pract. 93(Suppl 1):S2–S8. 10.1016/S0168-8227(11)70007-9 ^Gavin JR, et al. Insulin-dependent regulation of insulin receptor concentrations: a direct demonstration in cell culture. Proc Natl Acad Sci USA. 1974;71:84–88. ^Freychet P, Roth J, Neville DM., Jr Insulin receptors in the liver: specific binding of (125 I)insulin to the pla *** a membrane and its relation to insulin bioactivity. Proc Natl Acad Sci USA. 1971;68:1833–1837. ^Kasuga M, Zick Y, Blithe DL, Crettaz M, Kahn CR. Insulin stimulates tyrosine phosphorylation of the insulin receptor in a cell-free system. Nature. 1982;298:667–669. ^ Ebina Y, et al. The human insulin receptor cDNA: the structural basis for hormone-activated tran *** embrane signalling. Cell. 1985;40:747–758. ^Ullrich A, et al. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. Nature. 1985;313:756–761.^Ullrich A, et al. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. Nature. 1985;313:756–761.^Hubbard, S. R. (2013). The insulin receptor: both a prototypical and atypical receptor tyrosine kinase. Cold Spring Harbor Perspectives in Biology, 5, a008946^Anthony JC, Reiter AK, Anthony TG, Crozier SJ, Lang CH, MacLean DA, Kimball SR, and Jefferson LS. Orally administered leucine enhances protein synthesis in skeletal muscle of diabetic rats in the^Pham PT, Heydrick SJ, Fox HL, Kimball SR, Jefferson LS, and Lynch CJ. Asses *** ent of cell signaling pathways in the regulation of mTOR by amino acids in rat adipocytes. J Cell Biochem 79: 427–441, 2000.^Campbell LE, Wang X, and Proud CG. Nutrients differentially regulate multiple translation factors and their control by insulin. Biochem J 344: 433–441, 1999.^Anton J. M. Wagenmakers,corresponding author 1 ,* Juliette A. Strauss, 1 Sam O. Shepherd, 1 Michelle A. Keske, 2 and Matthew Cocks.Increased muscle blood supply and transendothelial nutrient and insulin transport induced by food intake and exercise: effect of obesity and ageing.2016 Apr 15; 594(8): 2207–2222.^Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR, Barrett EJ. Obesity blunts insulinmediated microvascular recruitment in human forearm muscle. Diabetes. 2006;55(5):1436-1442^Clerk LH, Vincent MA, Barrett EJ, Lankford MF, Lindner JR. Skeletal muscle capillary responses to insulin are abnormal in late-stage diabetes and are restored by angiotensinconverting enzyme inhibition. American journal of physiology Endocrinology and metaboli *** . 2007;293(6):E1804-1809.^Vincent MA, Clerk LH, Lindner JR, et al. Mixed meal and light exercise each recruit muscle capillaries in healthy humans. American journal of physiology Endocrinology and metaboli *** . 2006;290(6):E1191-1197.^Vincent MA, Clerk LH, Lindner JR, Price WJ, Jahn LA, Leong‐Poi H & Barrett EJ (2006). Mixed meal and light exercise each recruit muscle capillaries in healthy humans. Am J Physiol Endocrinol Metab 290, E1191–E1197.^Millward DJ, Odedra B, and Bates PC. The role of insulin,corticosterone and other factors in the acute recovery of muscleprotein synthesis on refeeding food-deprived rats. Biochem J216: 583–587, 1983.^Svanberg E, Zachrisson H, Ohlsson C, Iresjo BM, andLundholm KG. Role of insulin and IFG-I in activation of muscleprotein synthesis after oral feeding. Am J Physiol Endocrinol Metab 270: E614–E620, 1996.^ Preedy VR and Garlick PJ. The response of muscle proteinsynthesis to nutrient intake in postabsorptive rats: the role ofinsulin and amino acids. Biosci Rep 6: 177–183, 1986.^Millward DJ, Odedra B, and Bates PC. The role of insulin,corticosterone and other factors in the acute recovery of muscleprotein synthesis on refeeding food-deprived rats. Biochem J216: 583–587, 1983.^Svanberg E, Zachrisson H, Ohlsson C, Iresjo BM, andLundholm KG. Role of insulin and IFG-I in activation of muscleprotein synthesis after oral feeding. Am J Physiol Endocrinol Metab 270: E614–E620, 1996.^Alberti KG, Eckel RH, Grundy *** , et al. Harmonizing the metabolic syndrome: a joint interim statement of the international Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645.^Guallar-Castillon P, Perez RF, Lopez Garcia E, et al. Magnitude and management of metabolic syndrome in Spain in 2008–2010: the ENRICA study. Rev Esp Cardiol. 2014;67(5):367–373.^Prasad DS, Kabir Z, Dash AK, et al. Prevalence and risk factors for metabolic syndrome in Asian Indians: a community study from urban eastern India. J Cardiovasc Dis Res. 2012;3(3):204–211.^Ford ES, Li C, Zhao G, et al. Prevalence of the metabolic syndrome among U.S. adolescents using the definition from the International Diabetes Federation. Diabetes Care. 2008;31(3):587–589.^Dokas J., Chadt A., Nolden T., Himmelbauer H., Zierath J.R., Joost H.G. Conventional knockout of Tbc1d1 in mice impairs insulin- and AICAR-stimulated glucose uptake in skeletal muscle.Endocrinology.2013;154:3502–3514.^Charlotte Brøns,1,2 Christine B Jensen,1 Heidi Storgaard,1 Natalie J Hiscock,3 Andrew White,3 Julie S Appel,1 Stine Jacobsen,1 Emma Nilsson,1 Claus M Larsen,1 Arne Astrup,2 Bjørn Quistorff,4 and Allan Vaag1.Impact of short-term high-fat feeding on glucose and insulin metaboli *** in young healthy men.2009 May 15; 587(Pt 10): 2387–2397.^Park YW, Chang Y, Sung KC, Ryu S, Sung E, Kim WS. The sequential changes in the fasting pla *** a glucose levels within normoglycemic range predict type 2 diabetes in healthy, young men. Diabetes Res Clin Pract. 2006;73:329–335.^Siôn A Parry, Mark C Turner , Rachel M Woods, Lewis J James, Richard A Ferguson, Matthew Cocks, Katie L Whytock, Juliette A Strauss, Sam O Shepherd, Anton J M Wagenmakers, Gerrit van Hall, Carl J Hulston.High-Fat Overfeeding Impairs Peripheral Glucose Metaboli *** and Muscle Microvascular eNOS Ser1177 Phosphorylation.2020 Jan 1;105(1):dgz018^Trujillo ME, Scherer PE. Adipose tissue-derived factors: impact on health and disease. Endocr.Rev. 2006;27:762–778. ^Ferrannini E, Natali A, Bell P, Cavallo-Perin P, Lalic N, Mingrone G. Insulin resistance and hypersecretion in obesity. European Group for the Study of Insulin Resistance (EGIR) J Clin Invest. 1997;100:1166–1173.^Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care. 1994;17:961–969. ^Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122:481–486.^Koh-Banerjee P, Wang Y, Hu FB, Spiegelman D, Willett WC, Rimm EB. Changes in body weight and body fat distribution as risk factors for clinical diabetes in US men. Am J Epidemiol. 2004;159:1150–1159.^Olefsky JM, Kolterman OG, Scarlett JA. Insulin action and resistance in obesity and noninsulin-dependent type 2 diabetes mellitus. Am J Physiol. 1982;243:E15–E30.^Lazar MA. How obesity causes diabetes: not a tall tale. Science. 2005;307:373–375. ^Wang J, Obici S, Morgan K, Barzilai , Feng Z, Rossetti L. Overfeeding rapidly induces leptin and insulin resistance. Diabetes. 2001;50:2786–2791. ^Horton TJ, Drougas H, Brachey A, Reed GW, Peters JC, Hill JO. Fat and carbohydrate overfeeding in humans: different effects on energy storage. Am J Clin Nutr. 1995;62:19–29.^Chinayon S, Goldrick RB. Effects of overfeeding on carbohydrate tolerance, insulin secretion, esterification and lipolysis in healthy subjects. Horm Metab Res. 1978;10:182–186. ^Clore JN, Helm ST, Blackard WG. Loss of hepatic autoregulation after carbohydrate overfeeding in normal man. J Clin Invest. 1995;96:1967–1972. ^Kashiwagi A, Mott D, Bogardus C, Lillioja S, Reaven GM, Foley JE. The effects of short-term overfeeding on adipocyte metaboli *** in Pima Indians. Metaboli *** . 1985;34:364–370. ^Kolaczynski JW, Ohannesian JP, Considine RV, Marco CC, Caro JF. Response of leptin to short-term and prolonged overfeeding in humans. J Clin Endocrinol Metab. 1996;81:4162–4165. ^Oppert JM, Nadeau A, Tremblay A, Després JP, Thériault G, Dériaz O, Bouchard C. Pla *** a glucose, insulin, and glucagon before and after long-term overfeeding in identical twins. Metaboli *** . 1995;44:96–105.^Ravussin E, Schutz Y, Acheson KJ, Du *** et M, Bourquin L, Jéquier E. Short-term, mixed-diet overfeeding in man: no evidence for "luxuskonsumption". Am J Physiol. 1998;249:E470–E477. ^Fava F, Gitau R, Griffin BA, Gibson GR, Tuohy KM, Lovegrove JA. The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome ‘at-risk’ population. Int J Obes. 2013;37(2):216–223.^Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. ^Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101(44):15718–15723.^Deopurkar R, Ghanim H, Friedman J, Abuaysheh S, Sia CL, Mohanty P, Viswanathan P, Chaudhuri A, Dandona P. Differential effects of cream, glucose, and orange juice on inflammation, endotoxin, and the expression of Toll-like receptor-4 and suppressor of cytokine signaling-3. Diabetes Care. 2010;33(5):991–997.^Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–1481. ^Lassenius MI, Pietiläinen KH, Kaartinen K, Pussinen PJ, Syrjänen J, For *** lom C, Pörsti I, Rissanen A, Kaprio J, Mustonen J, Groop PH, Lehto M; FinnDiane Study Group . Bacterial endotoxin activity in human serum is associated with dyslipidemia, insulin resistance, obesity, and chronic inflammation. Diabetes Care. 2011;34(8):1809–1815.^Xu XH, Shah PK, Faure E, Equils O, Thomas L, Fishbein MC, Luthringer D, Xu XP, Rajavashisth TB, Yano J, Kaul S, Arditi M. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation. 2001;104(25):3103–3108. ^Gut *** ann T, Müller M, Carroll SF, MacKenzie RC, Wiese A, Seydel U. Dual role of lipopolysaccharide (LPS)-binding protein in neutralization of LPS and enhancement of LPS-induced activation of mononuclear cells. Infect Immun. 2001;69(11):6942–6950. ^Covert MW, Leung TH, Gaston JE, Baltimore D. Achieving stability of lipopolysaccharide-induced NF-kappaB activation. Science. 2005;309(5742):1854–1857. ^Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336(15):1066–1071.^Emanuelli B, Peraldi P, Filloux C, Chavey C, Freidinger K, Hilton DJ, Hotamisligil GS, Van Obberghen E. SOCS-3 inhibits insulin signaling and is up-regulated in response to tumor necrosis factor-alpha in the adipose tissue of obese mice. J Biol Chem. 2001;276(51):47944–47949.^Krebs DL, Hilton DJ. SOCS: physiological suppressors of cytokine signaling. J Cell Sci. 2000;113(Pt 16):2813–2819.^M. N. Rao, T. C. Neylan, C. Grunfeld, K. Mulligan, M. Schambelan, J.-M. Schwarz, Subchronic sleep restriction causes tissue-specific insulin resistance. J. Clin. Endocrinol. Metab. 100, 1664–1671 (2015).^A. Shechter, R. Rising, S. Wolfe, J. B. Albu, M.-P. St-Onge, Postprandial thermogenesis and substrate oxidation are unaffected by sleep restriction. Int. J. Obes. 38, 1153–1158 (2014).^M.-Y. Chien, L.-Y. Wang, H.-C. Chen, The relationship of sleep duration with obesity and sarcopenia in community-dwelling older *** s. Gerontology 61, 399–406 (2015).^M. Monico-Neto, S. Q. Giampá, K. S. Lee, C. M. de Melo, H. de Sá Souza, M. Dáttilo, P. A. Minali, P. H. Santos Prado, S. Tufik, M. T. de Mello, H. K. M. Antunes, Negative energy balance induced by paradoxical sleep deprivation causes multicompartmental changes in adipose tissue and skeletal muscle. Int. J. Endocrinol. 2015, 908159 (2015).^McGarry JD, Foster DW. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. 1980;49:395–420.^Berg JM, et al. Biochemistry. W.H. Freeman; 2012.^Miles JM, Nelson RH. Contribution of triglyceride-rich lipoproteins to pla *** a free fatty acids. Horm Metab Res. 2007;39:726.^Bruce CR, Brolin C, Turner N, Clea *** y ME, van der Leij FR, Cooney GJ, Kraegen EW. Overexpression of carnitine palmitoyltransferase I in skeletal muscle in vivo increases fatty acid oxidation and reduces triacylglycerol esterification. Am J Physiol Endocrinol Metab. 2007;292:E1231–1237.^McGarry J.D., Brown N.F. The mitochondrial carnitine palmitoyltransferase system. Eur. J. Biochem. 1997;244:1–14.^G KALNITSKY, D F TAPLEY.A sensitive method for estimation of oxaloacetate.Biochem J. 1958 Sep;70(1):28-34.^F. L. Breusch.The fate of oxaloacetic acid in different organs.Biochem J. 1939 Nov; 33(11): 1757–1770.^Krebs HA. The citric acid cycle and the Szent-Gyorgyi cycle in pigeon breast muscle. Biochem J. 1940;34(5):775–779.^D B TYLER.Effect of citric acid-cycle intermediates on oxaloacetate utilization and succinate oxidation.Biochem J. 1960 Aug;76(2):293-7.^Brosnan, J.T. Interorgan amino acid transport and its regulation. J. Nutr.133:2068S2072S, 2003.^Jungas, R.L, M.L. Halperin, and J.T. Brosnan. Qualitative *** ysis of amino acid oxidation and related gluconeogenesis in humans. Physiol. Rev. 72:419-448, 1992.^Cheek D, Holt A, Hill D, Talbert J. Skeletal muscle cell mass and growth: the concept of the deoxyribonucleic unit. Pediatr Res 5: 312–328, 1971.^Cheek DB. The control of cell mass and replication. The DNA unit–a personal 20-year study. Early Hum Dev 12: 211–239, 1985.^O’Connor RS, Pavlath GK, McCarthy JJ, Esser KA. Point:Counterpoint: Satellite cell addition is/is not obligatory for skeletal muscle hypertrophy. J Appl Physiol: 103: 1107, 2007.^Kadi F, Schjerling P, Andersen LL, Charifi N, Madsen JL, Christensen LR, Andersen JL. The effects of heavy resistance training and detraining on satellite cells in human skeletal muscles. J Physiol 558: 1005–1012, 2004.^Salleo, A., G. LaSpada, G. Falzea, M. G. Denaro, and R. Cicciarello. Response of satellite cells and muscle fibers to long-term compensatory hypertrophy. J. Submicrosc. Cytol. Pathol. 15: 929–940, 1983.^ Schiaffino, S., S. Pierobon Bormioli, and M. Aloisi. The fate of newly formed satellite cells during compensatory muscle hypertrophy. Virchows Arch. 21: 113–118, 1976.^Snow, M. H. Satellite cell response in rat soleus muscle undergoing hypertrophy due to surgical ablation of synergists. Anat. Rec. 227: 437–446, 1990.^Schultz, E., and K. M. McCormick. Skeletal muscle satellite cells. Rev. Physiol. Biochem. Pharmacol. 123: 213–257, 1994.^Molnar, G., M. L. Ho, and N. A. Schroedl. Evidence for multiple satellite cell populations and a non-myogenic cell type that is regulated differently in regenerating and growing skeletal muscle. Tissue Cell 28: 547–556, 1996.^Allen, D. L., S. R. Monke, R. J. Talmadge, R. R. Roy, and V. R. Edgerton. Plasticity of myonuclear number in hypertrophied and atrophied mammalian skeletal muscle fibers. J. Appl. Physiol. 78: 1969–1976, 1995.^Edgerton, V. R., and R. R. Roy. Regulation of skeletal muscle fiber size, shape and function. J. Biomechanics 24: 123–133, 1991.^Rosenblatt, J. D., D. Yong, and D. J. Parry. Satellite cell activity is required for hypertrophy of overloaded *** rat muscle. Muscle Nerve 17: 608–613, 1994.^Roy, R. R., K. M. Baldwin, T. P. Martin, S. P. Chimarusti, and V. R. Edgerton. Biochemical and physiological changes in overloaded rat fast- and slow-twitch ankle extensors. J. Appl. Physiol. 59: 639–646, 1985^Allen RE, Merkel RA, Young RB. Cellular aspects of muscle growth: myogenic cell proliferation. J Anim Sci 49: 115–127, 1979.^Edgerton, V. R., and R. R. Roy. Regulation of skeletal muscle fiber size, shape and function. J. Biomechanics 24, Suppl. 1: 123–133, 1991.^Anderson JE. Wozniak AC. Satellite cell activation on fibers: modeling events in vivo–an invited review. Can. J. Physiol. Pharmacol. 2004;82:300–310.^Donna M D’Souza,1,* Karin E Trajcevski,1,* Dhuha Al-Sajee,1 David C Wang,1 Melissa Thomas,1 Judy E Anderson,2 and Thomas J Hawke1.Diet-induced obesity impairs muscle satellite cell activation and muscle repair through alterations in hepatocyte growth factor signaling.Physiol Rep. 2015 Aug; 3(8): e12506.^Maffiuletti N.A., Ratel S., Sartorio A., Martin V. The Impact of Obesity on In Vivo Human Skeletal Muscle Function. Curr. Obes. Rep. 2013;2:251–260. ^Tomlinson D.J., Erskine R.M., Morse C.I., Winwood K., Onambélé-Pearson G. The impact of obesity on skeletal muscle strength and structure through adolescence to old age. Biogerontology. 2016;17:467–483. ^Bollinger L.M. Potential contributions of skeletal muscle contractile dysfunction to altered biomechanics in obesity. Gait Posture. 2017;56:100–107. ^Maffiuletti NA, Ratel S, Sartorio A, Martin V. The Impact of Obesity on In Vivo Human Skeletal Muscle Function. Curr Obes Rep 2: 251–260, 2013. ^Rolland Y, Lauwers-Cances V, Pahor M, Fillaux J, Grandjean H, Vellas B. Muscle strength in obese elderly women: effect of recreational physical activity in a cross-sectional study. Am J Clin Nutr 79: 552–557, 2004.^Pedersen A.N., Ovesen L., Schroll M., Avlund K., Era P. Body composition of 80-years old men and women and its relation to muscle strength, physical activity and functional ability. J. Nutr. Health Aging. 2002;6:413–420.^Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol. 2001;90(6):2157–65.^Visser M, Kritchevsky SB, Goodpaster BH, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50(5):897–904. ^Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60(3):324–33. ^Hilton TN, Tuttle LJ, Bohnert KL, Mueller MJ, Sinacore DR. Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: association with performance and function. Phys Ther. 2008;88(11):1336–44.^ Lawler JM, Song W, Demaree SR. Hindlimb unloading increases oxidative stress and disrupts antioxidant capacity in skeletal muscle. Free Radic Biol Med. 2003;35:9–16.^Ruan XY, Gallagher D, Harris T, et al. Estimating whole body intermuscular adipose tissue from single cross-sectional magnetic resonance images. J Appl Physiol. 2007;102:748–754. ^Choi S.J., Files D.C., Zhang T., Wang Z.M., Messi M.L., Gregory H., Stone J., Lyles M.F., Dhar S., Marsh A.P., et al. Intramyocellular Lipid and Impaired Myofiber Contraction in Normal Weight and Obese Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2016;71:557–564. ^N Miyatake 1, M Fujii, H Nishikawa, J Wada, K Shikata, H Makino, I Kimura.Clinical evaluation of muscle strength in 20-79-years-old obese Japanese.Diabetes Res Clin Pract. 2000 Apr;48(1):15-21.^M Hulens 1, G Vansant, R Lysens, A L Claessens, E Muls, S Brumagne.Study of differences in peripheral muscle strength of lean versus obese women: an allometric approach.Int J Obes Relat Metab Disord . 2001 May;25(5):676-81.^abPaolillo F.R., Milan J.C., Bueno Pde G., Paolillo A.R., Borghi-Silva A., Parizotto N.A., Arena R., Kurachi C., Bagnato V.S. Effects of excess body mass on strength and fatigability of quadriceps in postmenopausal women. Menopause. 2012;19:556–561. ^Ochala J, Frontera WR, Dorer DJ, Van Hoecke J, Krivickas LS. Single skeletal muscle fiber elastic and contractile characteristics in young and older men. J Gerontol A Biol Sci Med Sci. 2007;62:375–381.^Tikunov BA, Sweeney HL, Rome LC. Quantitative electrophoretic *** ysis of myosin heavy chains in single muscle fibers. J Appl Physiol (1985). 2001;90:1927–1935. ^Rahemi H, Nigam N, Wakeling JM. The effect of intramuscular fat on skeletal muscle mechanics: implications for the elderly and obese. J R Soc Interface. 2015;12:125–129. ^Hilton TN, Tuttle LJ, Bohnert KL, Mueller MJ, Sinacore DR. Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: association with performance and function. Phys Ther. 2008;88:1336–1344.^ Nicola A Maffiuletti 1, Marc Jubeau, Urs Munzinger, Mario Bizzini, Fiorenza Agosti, Alessandra De Col, Claudio L Lafortuna, Alessandro Sartorio.Differences in quadriceps muscle strength and fatigue between lean and obese subjects.Eur J Appl Physiol. 2007 Sep;101(1):51-9.^O’Neill HM, Holloway GP, Steinberg GR. AMPK regulation of fatty acid metaboli *** and mitochondrial biogenesis: implications for obesity. Mol Cell Endocrinol 366: 135–151, 2013.^Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 8: 1288 – 1295, 2002. ^Huxley AF, Niedergerke R (1954) Structural changes in muscle during contraction. Interference microscopy of living muscle fibres. Nature 173:971–973.^Huxley HE, Hanson J (1954) Changes in cross-striations of muscle during contraction and stretch and their structural implications. Nature 173:973–976.^Huxley HE (1969) The mechani *** of muscular contraction. Science 164:1356–1366.^Huxley AF, Simmons RM (1971) Proposed mechani *** of force generation in striated muscle.^Rayment I, Holden HM, Whittaker M, Yohn CB, Lorenz M, Holmes KC, Milligan RA (1993) Structure of the actin-myosin complex and its implications for muscle contraction. Science 261:58–65.^Rayment I, Rypniewski WR, Schmidt-B?se K, Smith R, Tomchick DR, Benning MM, Winkelmann DA, Wesenberg G, Holden HM (1993) Three-dimensional structure of myosin subfragment-1: a molecular motor. Science 261:50–58.^Eisenberg E, Hill TL. Muscle contraction and free energy transduction in biological systems. Science. 1985 Mar 1;227(4690):999–1006.^Eisenberg E, Hill TL, Chen Y. Cross-bridge model of muscle contraction. Quantitative *** ysis. Biophys J. 1980 Feb;29(2):195–227.^Finer JT, Simmons RM, Spudich JA. Single myosin molecule mechanics: piconewton forces and nanometre steps. Nature. 1994 Mar 10;368(6467):113–119.^Thorson J, White DC. Distributed representations for actin-myosin interaction in the oscillatory contraction of muscle. Biophys J. 1969 Mar;9(3):360–390.^Wakabayashi K, Sugimoto Y, Tanaka H, Ueno Y, Takezawa Y, Amemiya Y. X-ray diffraction evidence for the extensibility of actin and myosin filaments during muscle contraction. Biophys J. 1994 Dec;67(6):2422–2435.^R D Bremel, A Weber.Cooperation within actin filament in vertebrate skeletal muscle.Nat New Biol. 1972 Jul 26;238(82):97-101.^Ricarda Haeger , Felipe de Souza Leite , Dilson E Rassier.Sarcomere length non-uniformities dictate force production along the descending limb of the force-length relation.Proc Biol Sci. 2020 Oct 28;287(1937):20202133.^Dilson E Rassier.Sarcomere mechanics in striated muscles: from molecules to sarcomeres to http://cells.Am J Physiol Cell Physiol. 2017 Aug 1;313(2):C134-C145.Epub 2017 May 24.^D A Smith.The theory of sliding filament models for muscle contraction. III. Dynamics of the five-state model.J Theor Biol. 1990 Oct 21;146(4):433-66.^B Brenner, M Schoenberg, J M Chalovich, L E Greene, E Eisenberg.Evidence for cross-bridge attachment in relaxed muscle at low ionic strength.Proc Natl Acad Sci U S A. 1982 Dec;79(23):7288-91.^Kiisa Nishikawa 1 , Samrat Dutta 2 , Michael DuVall 2 3 , Brent Nelson 4 , Matthew J Gage 5 , Jenna A Monroy 6.Calcium-dependent titin-thin filament interactions in muscle: observations and theory.J Muscle Res Cell Motil. 2020 Mar;41(1):125-139.Epub 2019 Jul 9.^B Brenner, E Eisenberg.The mechani *** of muscle contraction. Biochemical, mechanical, and structural approaches to elucidate cross-bridge action in muscle.Basic Res Cardiol. 1987;82 Suppl 2:3-16.^J C Haselgrove, H E Huxley.X-ray evidence for radial cross-bridge movement and for the sliding filament model in actively contracting skeletal muscle.J Mol Biol. 1973 Jul 15;77(4):549-68.^R Dabrowska, W Drabikowski.Molecular basis of muscular contraction.Postepy Biochem. 1973;19(3):343-59.^Gerald H. Pollack.On the Contractile Mechani *** in Cardiac Muscle.Cardiac Electrophysiology, Circulation.^T R Leonard 1 , W Herzog.Regulation of muscle force in the absence of actin-myosin-based cross-bridge interaction. 15: 448-54 Physiol Cell Physiol. 2010 Jul;299(1):C14-20. Epub 2010 Mar 31.^Bruton JD, Katz A, Lännergren J, Abbate F, Westerblad H. Regulation of myopla *** ic Ca(2) in genetically obese (ob/ob) mouse single skeletal muscle fibres. Pflugers Arch 444: 692–699, 2002. ^Ciapaite J, van den Berg SA, Houten *** , Nicolay K, van Dijk KW, Jeneson JA. Fiber-type-specific sensitivities and phenotypic adaptations to dietary fat overload differentially impact fast- versus slow-twitch muscle contractile function in C57BL/6J mice. J Nutr Biochem 26: 155–164, 2015. ^Benjamin T Wall 1, Marlou L Dirks 2, Tim Snijders 2, Jan-Willem van Dijk 1, Mario Fritsch 3, Lex B Verdijk 1, Luc J C van Loon 4.Short-term muscle disuse lowers myofibrillar protein synthesis rates and induces anabolic resistance to protein ingestion.Am J Physiol Endocrinol Metab. 2016 Jan 15;310(2):E137-47. ^Benjamin T Wall 1, Tim Snijders, Joan M G Senden, Chris L P Ottenbros, Annemie P Gijsen, Lex B Verdijk, Luc J C van Loon.Disuse impairs the muscle protein synthetic response to protein ingestion in healthy men.J Clin Endocrinol Metab. 2013 Dec;98(12):4872-81.^Sean P Kilroe 1, Jonathan Fulford 2, Andrew M Holwerda 3, Sarah R Jackman 1, Benjamin P Lee 4, Annemie P Gijsen 3, Luc J C van Loon 3, Benjamin T Wall 1.Short-term muscle disuse induces a rapid and sustained decline in daily myofibrillar protein synthesis rates.Am J Physiol Endocrinol Metab . 2020 Feb 1;318(2):E117-E130. ^Benjamin T Wall 1, Tim Snijders, Joan M G Senden, Chris L P Ottenbros, Annemie P Gijsen, Lex B Verdijk, Luc J C van Loon.Disuse impairs the muscle protein synthetic response to protein ingestion in healthy men.J Clin Endocrinol Metab. 2013 Dec;98(12):4872-81.